HELP Releases Wide-Ranging Health Care Cost Legislation
Leadership of the Senate Health, Education, Labor, and Pensions (HELP) Committee released a draft bipartisan health care package that aims to contain health costs. The bill includes measures to address surprise insurance gaps, reduce the cost of prescription drugs, create more transparency in the health care system, improve public health, and increase health information exchange.
Under the legislation, patients would be required to pay only their in-network cost-sharing amount for out-of-network emergency care and treatments provided by ancillary out-of-network providers. The proposal outlines three options for resolving payment disputes between providers and insurers: an in-network guarantee under which providers can join a health plan’s network or bill through the facility where they practice; use of an independent dispute resolution process; or payment based on the median contracted rate for the services rendered. States would have the option of enacting or continuing with current state laws or regulations. If a patient is stabilized after entering a facility through the emergency department, the patient must be given advance notice of any out-of-network care, an estimate of the cost, and referrals for alternative options for in-network care. Health plans and providers would also be required to provide patients with estimates of their out-of-pocket costs for a service within 48 hours of a request, and to send any medical bills within 30 days of the service. Insurers would be mandated to keep their provider directories up to date.
In regard to rising drug costs, the bill would ban the practice of spread pricing by pharmacy benefit managers (PBMs) and require that PBMs provide quarterly reports on costs, fees, and rebates. PBMs would also be required to pass on 100 percent of the rebates negotiated to their client. The HELP proposal would prevent the Food and Drug Administration (FDA) from delaying applications for new insulin products. It also includes provisions to update drug patent registries, increase competition by preventing generic manufacturers from blocking competitors from coming to market, and give the FDA more flexibility in how the agency deals with petitions from brand manufacturers.
The package would prohibit gap clauses between providers and insurers that hide cost and quality information from consumers, as well as anti-tiering and anti-steering clauses in contracts between providers and health plans. It includes funding for programs related to vaccine education, maternal mortality, health care professional discrimination and bias training, and to improve the privacy and security of health information and electronic medical records.
Chairman Alexander has indicated that he is working with members of his panel to add a provision to tie patient costs to a drug’s negotiated price rather than its list price, and a measure to require more detailed disclosures from pharmaceutical manufacturers. The HELP Committee plans to hold a hearing on the discussion draft in June before marking it up prior to July 4. The current plan is for the bill to be combined with legislation being drafted by the Finance Committee before being brought to the Senate floor in July.
Prescription Drug Legislation Continues in Senate
While the President’s declaration may possibly threaten bipartisan negotiations between Congress and the White House on drug pricing, Senate Finance Committee Chairman Chuck Grassley (R-Iowa) has stated that work to strike a bipartisan deal among members of his panel continues. His package, which is expected to be unveiled sometime in June, will focus on increasing generic competition and possibly include a cap on seniors’ Part D out-of-pocket expenses. Senator Grassley has previously criticized measures to allow Medicare to negotiate prices in Part D, an idea that was being discussed by House Speaker Nancy Pelosi (D-Calif.) in her talks with the White House.
Senate Finance Continues Insulin Pricing Investigation
Leadership of the Senate Finance Committee have requested information about federal spending on insulin and average out-of-pocket costs for beneficiaries as a part of their investigation into the rising cost of insulin. Chairman Chuck Grassley (R-Iowa) and Ranking Member Ron Wyden (D-Ore.) wrote to Centers for Medicare and Medicaid Services (CMS) Administrator Seema Verma to ask for data on the impact of insulin prices and other diabetes-related costs on both patients and federal health care program spending. The lawmakers also ask for details on how much money pharmacy benefit managers (PBMs) make from insulin rebates and the administrative fees paid by insulin manufacturers.
New MedPAC, MACPAC Commissioners Announced
The Government Accountability Office (GAO) has announced the appointment of two new members to the Medicare Payment Advisory Commission (MedPAC): Lawrence Casalino, MD, PhD, Livingston Professor of Public Health and Chief of the Division of Healthcare Policy and Economics in the Weill Cornell Department of Healthcare Policy and Research, and Amol Navathe, MD, PHD, co-Director of the Healthcare Transformation Institute and Associate Director of the Center for Health Incentives and Behavioral Economics in the Department of Medical Ethics and Health Policy at the University of Pennsylvania School of Medicine. Their terms will expire in April 2022. The newly appointed MedPAC vice- chair will be Paul Ginsburg, PhD.
The GAO also announced the new members that will be joining the Medicaid and CHIP Payment and Access Commission (MACPAC): Tricia Brooks of Georgetown University and Thomas Barker of Foley Hoag. Melanie Bella, Cityblock Health, was named the new MACPAC chair, while Charles Miligan, UnitedHealthcare, will serve as vice-chair.
Senate Passes Health Care Bills by Unanimous Consent
The Senate passed two pieces of health care related legislation by voice vote last week. S. 1379, the Pandemic and All-Hazards Preparedness and Advancing Innovation Act, would reauthorize programs related to emergency preparedness and public health response efforts. S. 820, the Debbie Smith Act of 2019, would strengthen programs dealing with DNA testing of evidence related to sexual assaults.
Lawmakers Push for DSH Cut Delay
A group of 300 bipartisan lawmakers have written to House leadership pushing for a delay of Medicaid disproportionate share hospital (DSH) cuts. The DSH funding cuts have already been delayed several times since the passage of the ACA and are now scheduled to go into effect on Oct. 1. The letter, which was led by Reps. Eliot Engel (D N.Y.) and Pete Olson (R-Texas), calls for the $4 billion in cuts to be delayed for at least two years, or “until a more sustainable, permanent solution is reached.” On the Senate side, Finance Committee Chairman Chuck Grassley (R-Iowa) has previously expressed interest in a permanent solution to reforming the safety net hospital payment system.
Finance Leaders Consider OOP Cap
Senate Finance Committee Chairman Chuck Grassley (R-Iowa) has said that he is working with Ranking Member Ron Wyden (D-Ore.) on a bipartisan plan to cap out-of-pocket (OOP) expenses for prescription drugs in the Medicare program. The two lawmakers are reportedly also in discussions about drug pricing and incentive changes in Medicare Part D, Part B, and Medicaid.
President Pushes Importation Proposal
President Trump has endorsed a proposal from Florida Gov. Ron DeSantis (R) that would allow the state to import prescription drugs from Canada. In order for Florida’s importation policy to go into effect, the Secretary of the U.S. Department of Health and Human Services (HHS) would have to certify that the imported drugs would be safe, a move that no HHS secretary has made before. Key officials have recently raised concerns with the safety of the importation plan, including the Secretary of the Department of Health and Human Services (HHS) Alex Azar and Senate Health, Education, Labor, and Pensions (HELP) Committee Chairman Lamar Alexander (R-Tenn.).
Appropriators Release Labor-HHS Committee Report
House appropriators released its report to accompany the committee’s FY 2020 Labor-HHS-Education spending bill. In the report, lawmakers raise concerns about the recent proposed rule from the Centers for Medicare and Medicaid Services (CMS) that would allow health plans to exclude six protected classes of drugs from Medicare Part D formularies and to make greater use of utilization management tools like step therapy and prior authorization.
The committee report states that such changes could negatively impact seniors access to Part D drugs and critical therapies for HIV, cancer, and mental illness. It directs CMS to increase the use of electronic prior authorization, require Medicare Advantage (MA) plans to rein in prior authorization requirements, and to issue guidance preventing plans from excluding coverage of services that “that align with evidence-based guidelines and have historically high prior-authorization approval rates.” The guidance would also require MA insurers to submit an annual list of things for which they demand prior- authorization to the secretary of the HHS. CMS is also asked to consider testing health benefit designs that eliminate specialty tiers to increase the affordability of gene therapies and biologic treatments, particularly for rare diseases.
In a related section, the report asks the administration to consider reducing patient copays and incentivizing providers to increase the utilization of biosimilars in Medicare Part B. The report requests that the administration submit information on prescription drug price changes in government health programs since 2008. Appropriators ask for a list of drugs that have been approved in the past five years that have benefited significantly from government grants or research subsidies. A report on Medicare drug spending, detailing total spending on certain drugs and their net prices, is also requested.
The committee report includes language regarding medical workforce shortages. It recommends that CMS convene relevant stakeholders to review changes in population health and prepare a report for the Committee. Lawmakers also encourage the HHS secretary to allow teaching clinicians to verify their nurse practitioner and physician assistant student’ notes during their evaluation rather than having to re-document the information. CMS is also asked to explain how it is evaluating nurse hospital staffing levels.
President Urges Congress to Act on Surprise Medical Bills
President Trump has called on Congress to address the issue of surprise medical bills and ban the practice of balance billing. The President did not endorse any specific legislation, but instead laid out the administration’s general principles for future legislation, stating that patients should not incur out-of-network costs from hospitals and doctors for emergency services, and that patients should not get out-of-network bills from a provider they did not pick. In cases of nonemergency treatment, patients should be informed in advance if any of the care will be out-of-network and the costs that they will face. The White House also specifies that any legislative solution should not increase federal health care expenditures. The position statement does not specify how billing disputes between insurers and providers should be solved, but White House officials have raised concerns about the potential disruption caused by arbitration and the patient abuses that could result.
Sen. Bill Cassidy (R-La.), who is leading a bipartisan working group on the issue, has spoken to the positive track record of arbitration on a state level, but has said that he is also open to other ideas. Cassidy, along with Sen. Maggie Hassan (D-N.H.), are still in the process of determining in what direction to take the bill. Following the President’s speech, leadership from all three House committees with health jurisdiction released bipartisan statements with the intent to work together on the issue. House Energy and Commerce Chairman Frank Pallone (D-N.J.) and Ranking Member Greg Walden (R-Ore.) stated that they are working together on bipartisan solution to protect patients, which they hope to announce soon. Senate Health, Education, Labor, and Pensions Committee Chairman Lamar Alexander (R-Tenn.) told the President that he hopes to present a bill in July.
Doc Caucus Urges Administration to Reconsider Arbitration
The House GOP Doctors Caucus sent a letter to HHS Secretary Alex Azar, Director of the Domestic Policy Council Joe Grogan, and Labor Secretary Alexander Acosta regarding surprise insurance gaps.
The letter praises the administration for acknowledging surprise medical billing as a significant problem facing American patients and urges the White House officials to look toward successful state models such as the ones enacted in New York and Maryland. The lawmakers argue that the arbitration model based on a third-party charge system is the most appropriate way to address surprise insurance gaps, and request that the administration “reconsider its comments that arbitration would be a disruptive and unnecessary distraction.” The letter also highlights the Caucus’s concerns with bundled billing and network matching approaches.
Judge Rules Against Cuts to 340B
The federal court has ruled that the Trump administration’s move to cut nearly 30 percent from the 340B drug discount program is unlawful. The lawsuit was brought by the American Hospital Association, America’s Essential Hospitals, the Association of American Medical Colleges, and three hospitals. HHS has until August 5 to submit a status report to U.S. District Court Judge Rudolph Contreras about how the department is remedying the situation
CBO Releases Highly Anticipated Report on Single-Payer Health Care
The CBO released its report on single-payer health care. The report, requested by House Budget Chairman John Yarmuth (D-Ky.), does not make any recommendations but is instead an analysis of the potential impact of switching to a single-payer health care system. It examines what types of services would be covered, how people would enroll, the kinds of decisions that lawmakers would have to make in crafting such a policy, and the potential budgetary and economic impact of such legislative proposals. It does not include any formal consideration or score of Medicare for All legislation or estimates of how many people would gain or lose coverage under the system currently being advocated by Sen. Bernie Sanders (I-Vt.) and Rep. Pramila Jayapal (D-Wash.). CBO states that government spending on health care under a single-payer system would “increase substantially” and outlines the significant economic disruptions that could occur if the switch were to be made. Substantial changes would result in the sources and extent of health care coverage, provider payment rates, and the financing of the nation’s health care.
The report warns of the increased demand for services and the possible added pressure of a single-payer system on providers. The budget office states that disruptions could be minimized by phasing in changes gradually. CBO also includes a high-level discussion of options to finance prescription drugs in a single-payer system, such as direct negotiation with the manufacturer. Chairman Yarmuth has expressed hopes that the report will accelerate the move to a single-payer system, which he has described as inevitable, while Ranking Member Steve Womack (R-Ark.) used the report to highlight the potential negative impacts of a “one size-fits-all” approach to health care. The House Budget Committee held a hearing on single-payer health care focusing on the recent CBO report. The House Ways and Means Committee has also confirmed plans to hold a hearing on single- payer health care. Leadership of the House Energy and Commerce Committee, the other House panel with health care jurisdiction, has not committed to hold a hearing on the issue but has instead reiterated a commitment focusing on legislation to lower health care costs.
CBO Details Cost of WH Rebate Rule
The CBO released a new analysis indicating that the administration’s plan to eliminate drug-manufacturer rebates to PBMs in Medicare and Medicaid would increase federal spending by $177 billion over the next decade. The White House proposal would significantly curtail the use of rebates between pharmaceutical companies and PBMs. CBO found that the move would increase spending for both the Medicare ($170 billion) and Medicaid ($7 billion) programs. The estimated increase in cost would be a result of higher insurance premiums – rebates can currently be used to broadly lower premiums. CBO also found that the rebate proposal would result in lower out-of-pocket costs for some Medicare beneficiaries, which could lead to higher drug utilization. CBO predicts that the policy would lead to manufacturers withholding 15 percent of the amounts they currently rebate to PBMs in Medicare and Part D. House Speaker Nancy Pelosi (D-Calif.) has reportedly asked the CBO to determine how much would be saved by delaying the rebate proposal. A delay or full repeal of the policy could be combined with other Democratic drug pricing proposals, such as capping Medicare out-of-pocket costs for prescriptions and then included as a part of must-pass legislation, like raising the budget caps.
CBO Releases Updated Estimates of ACA Mandate Repeal
The CBO estimates that the repeal of the Affordable Care Act’s (ACA) individual mandate will result in seven million more people without health insurance by 2020. The agency’s latest report projects that the number of uninsured will continue to grow – from 28 million in 2017 to 35 million by 2029. CBO attributes this increase not only to the repeal of the individual mandate but also to rising health insurance premiums and an increase in the number of people choosing plans that do not meet the definition of health insurance (short-term, limited-duration plans). Subsidies in the ACA marketplace are estimated to increase from $62 billion in 2019 to $79 billion in 2029, while the number of people who receive subsidies is projected to drop from eight million people to six million people. CBO’s most recent projections are similar to those made by the budget office in 2018.
Lawmakers Urge Continued Delay of New OPTN Policy
Sens. Roy Bunt (R-Mo.) and Jerry Moran (R-Kan.) have written to HHS Secretary Azar asking him to continue to delay changes made to the national liver distribution policy by the Organ Procurement and Transplantation Network (OPTN), pending additional information that the lawmakers have requested from the Government Accountability Office (GAO). Blunt and Moran have expressed concerns about the potential for the new liver allocation policy to bias other organ distribution policies, and about the lack of adequate oversight of changes made by the OPTN.
Stopping Surprise Billing Legislation
Reacting to the increase in reported surprise billing incidents in the media, Congress is releasing legislation to stop the practice. There are three instances where this occurs: emergency care, in-network facility with out-of-network providers, and in-network provider at an out-of-network facility. In all these instances the goal is to remove the patient from the billing dispute between the insurance company and the provider. It is at that point where the hair splitting begins in the proposed legislation as to who gets paid, how can disputes be settled, and what modes and methods are allowed.
The following is a brief outline of each of the proposed legislation currently being discussed. At the end of the legislation is a brief primer on the payment methods.
No Surprises Act
House Energy and Commerce Committee leadership released a bipartisan draft bill to address the issue of surprise insurance gaps during a stakeholder meeting, making it the first piece of surprise billing legislation from a committee with health care jurisdiction this year. The proposal, unveiled by Chairman Frank Pallone (D-N.J.) and Ranking Member Greg Walden (R-Ore.), is in line with the call for action and principles previously outlined by the White House. It would require hospitals and physicians to notify patients both in writing and orally when they are going to be treated by an out-of- network provider. The bill would prohibit surprise charges for emergency care and require insurers to treat out-of-network emergency care as in-network for the purpose of enrollee cost-sharing and out-of-pocket obligations.
The No Surprises Act draft would rely upon negotiated in-network rates to resolve out-of-network payment disputes. It would also provide $50 million in grants for states seeking to develop or maintain an all-payer claims database. The Committee is planning to schedule a hearing related to the legislation.
STOP Surprise Medical Bills Act
Bipartisan legislation on surprise billing (S. 1531) was also introduced in the Senate. The STOP Surprise Medical Bills Act comes after almost a year of work from a bipartisan group of senators led by Sens. Bill Cassidy (R-La.) and Maggie Hassan (D-N.H.). While the legislation is similar to the House proposal, the bills differ in the approach taken to payment disputes.
Under the Senate bill, providers would be paid the difference between a patient’s in-network cost sharing amount and the median in-network rate for the service, but both providers and insurers would have the opportunity to appeal the payment amount through an independent dispute resolution process in which an arbiter, certified by the secretaries of Health and Human Services and the Department of Labor, must choose between the two offers. The final arbitration decision would be based upon commercially-reasonable rates for the geographic area. Patients would only owe their in network rate for the services in question, in line with the House legislation. States that have already established an alternative mechanism to address surprise insurance gaps and determine payment amounts would be able to continue with those systems under certain circumstances.
Senate HELP Draft
In the Senate Health, Education, Labor, and Pensions (HELP) Committee draft bipartisan health care package, measures are included to address surprise insurance gaps.
Under the legislation, patients would be required to pay only their in-network cost-sharing amount for out-of-network emergency care and treatments provided by ancillary out-of-network providers. The proposal outlines three options for resolving payment disputes between providers and insurers: an in-network guarantee under which providers can join a health plan’s network or bill through the facility where they practice; use of an independent dispute resolution process; or payment based on the median contracted rate for the services rendered. States would have the option of enacting or continuing with current state laws or regulations. If a patient is stabilized after entering a facility through the emergency department, the patient must be given advance notice of any out-of-network care, an estimate of the cost, and referrals for alternative options for in-network care. Health plans and providers would also be required to provide patients with estimates of their out-of-pocket costs for a service within 48 hours of a request, and to send any medical bills within 30 days of the service. Insurers would be mandated to keep their provider directories up to date.
Protecting People from Surprise Medical Bills Act
The Protecting People from Surprise Medical Bills Act was introduced by Representatives Raul Ruiz, M.D., (D-CA) and Phil Roe, M.D. (R-TN) follows along the previously introduced bills with regards to removing the patient from the billing dispute. The Act takes an approach to protecting patients from surprise bills by adopting an arbitration model. Under this model – similar to the one adopted by New York State in 2015 – if providers and insurers cannot agree on a payment rate, they can engage in an independent dispute resolution process (IDR). Under IDR, insurers and out-of-network providers each identify a cost for the patient’s care, and a neutral arbiter chooses the fairer price. This model creates an incentive for both parties to choose reasonable numbers to cover the cost of treatment.
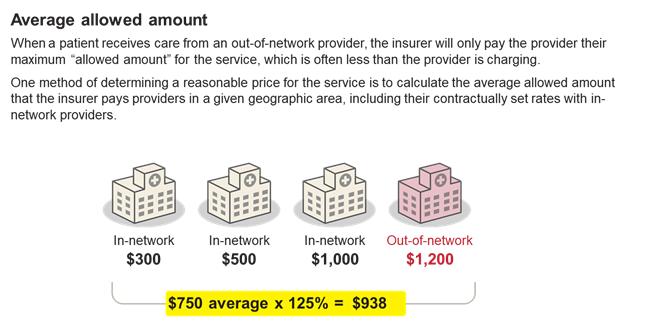
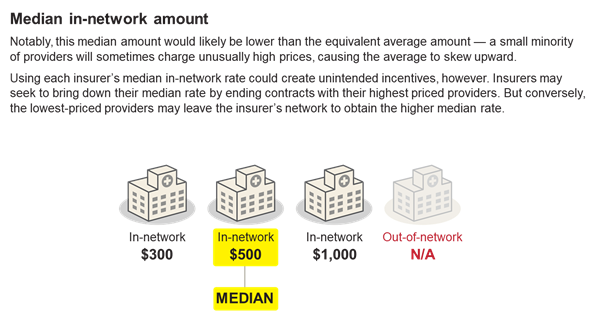
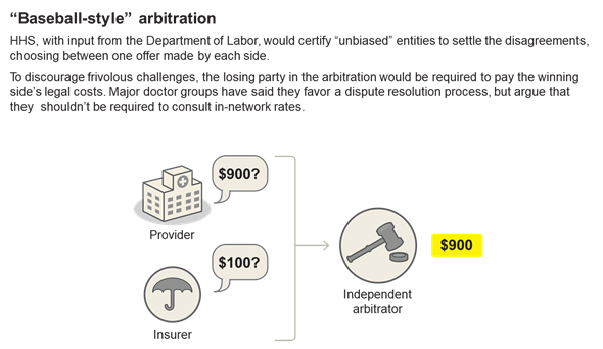
Payment Method Primer